
One mole of an ideal gas at standard temperature and pressure occupies 22.4 L (molar volume). What is the ratio of molar volume to the atomic volume of a mole of hydrogen ? (

Hydrogen, The Energy Of The Future, Is Composed Of One Atom Of Water And One Of Methane. H2. Possible Source Of Energy Stock Illustration - Illustration of energy, industry: 213117206
The weight of 350ml of diatomic gas at 0°C and 2 atm pressure is 1 g. The weight of one atom is? - Quora

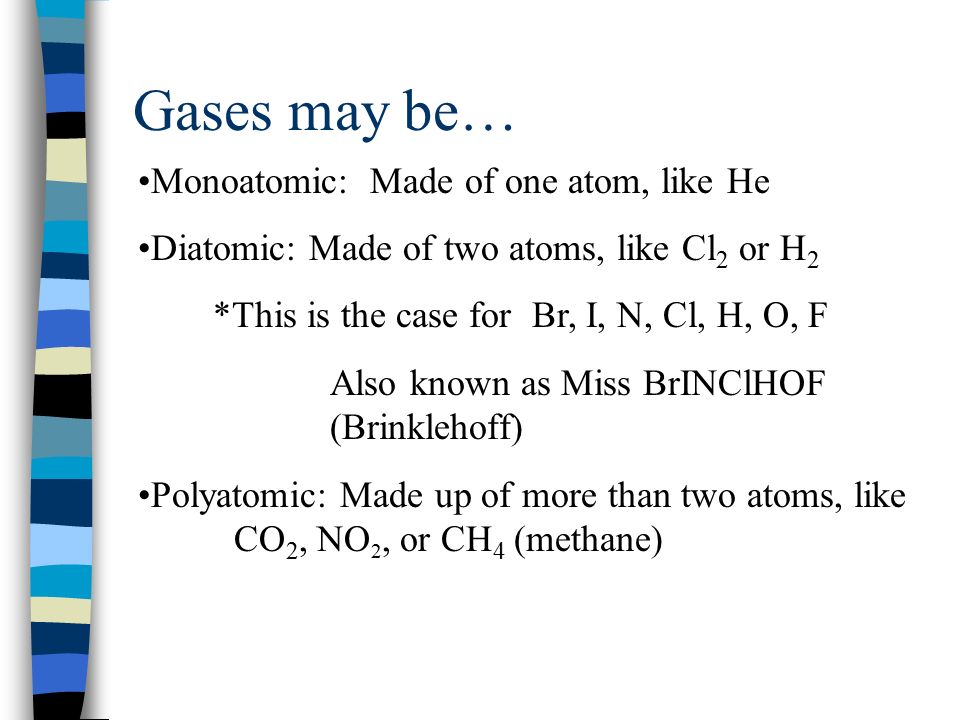
Monoatomic Gas: Made of one atom, like HeMonoatomic Gas: Made of one atom, like He Diatomic Gas: Made of two atoms, like Cl 2 or H 2Diatomic Gas: Made. - ppt download

The weight of `350mL` of a diatomic gas at `0^(@)C` and 2 atm pressure is `1 g`. The weight in - YouTube

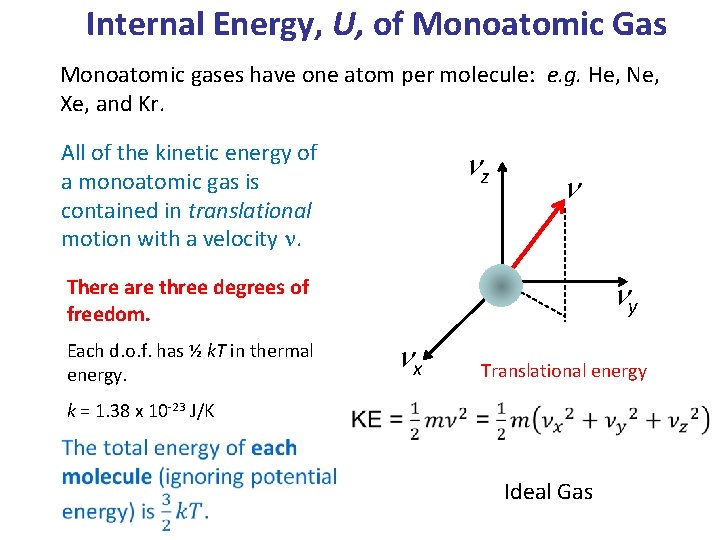
Chapter 14 The Ideal Gas Law and Kinetic Theory. To facilitate comparison of the mass of one atom with another, a mass scale know as the atomic mass scale. - ppt download








/GettyImages-545864473-5a769a63119fa8003740347a.jpg)





/186450350-56a132cb5f9b58b7d0bcf751.jpg)
